Difference Between Yellow Tungstic Acid and White Tungstic Acid
.jpg)
The difference between yellow tungstic acid and white tungstic acid firstly lies in the appearance, one in yellow, and the other in white; further, both of them have something difference in component, chemical property and generation mechanism. Yellow tungstic acid is the monohydrate with formula of WO3.H2O, while white tungstic acid is the dehydrate with formula of WO3.2H2O.
Yellow tungstic acid and white tungstic acid difference is showed as the following table:
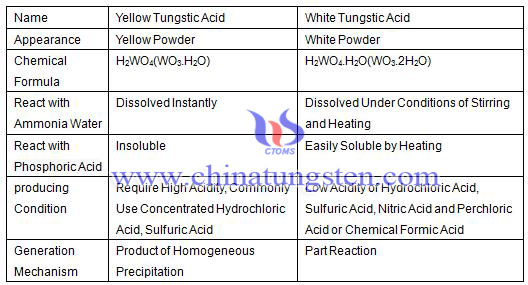
Generation Mechanism of Yellow Tungstic Acid and White Tungstic Acid
The generation mechanism of yellow tungstic acid and white tungstic acid is different, but they often intertwining in the generation.
Yellow powdery tungstic acid is a product of homogeneous precipitation, the breakdown products of contained tungsten complex anion, so its production is commonly using system of concentrated hydrochloric acid, while sulfuric acid can also achieve the same effect.
White tungstic acid can be generated at the low acidity of adding hydrochloric, sulfuric, nitric acid perchloric or chemical formic acid into sodium tungstate (Na2WO4) will all produce white colloidal tungstic acid, then, with the stirring, a higher grade of poly acid ion will be obtained and to produce colloidal white tungstic acid. In addition, adding sodium tungstate solution into dilute nitric acid will also manufacture powdery white tungstic acid.







