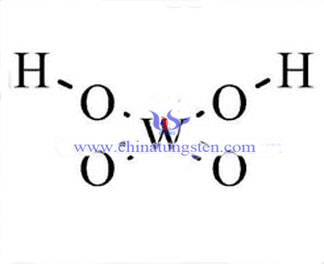
Tungstic Acid Molecular Structure

Tungsten acid molecular structure refers to positions of various constituent atoms in tungstic acid in space, including its bonding type, length, angle of chemical bond and dihedral angle between adjacent three keys.
Molecular structure is also known as molecular geometry, the three-dimensional arrangement of the atoms that constitute a molecule. It determines several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism, and biological activity. The angles between bonds that an atom forms depend only weakly on the rest of molecule, i.e. they can be understood as approximately local and hence transferable properties.
The molecular geometry can be determined by various spectroscopic methods and diffraction methods. Such as IR, microwave and Raman spectroscopy can give information about the molecule geometry from the details of the vibrational and rotational absorbance detected by these techniques; X-ray crystallography, neutron diffraction and electron diffraction can give molecular structure for crystalline solids based on the distance between nuclei and concentration of electron density; gas electron diffraction can be used for small molecules in the gas phase ect..
.