Tungstic Acid Synthetic Method

Tungstic acid synthetic method includes calcium tungstate and other tungstate hydrochloric acid decomposition method.
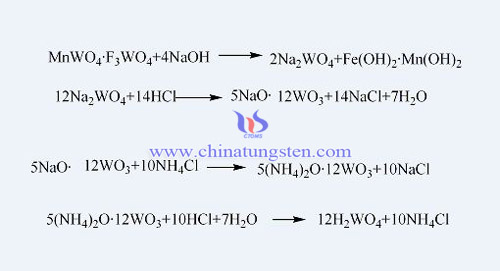
1. Calcium tungstate hydrochloric acid decomposition method, which is to generate sodium tungstate after the tungsten concentrate was broken out and reacted with sodium hydroxide, then the sodium tungstate was carried out filtration, purification, and went on metathesis reaction with calcium chloride solution to obtain calcium tungstate, and then, the calcium tungstate was taken out a treatment with hydrochloric acid to generate tungstic acid, finally we get product of tungstic acid after washing, drying and crushing. In the hydrochloric acid decomposition method, after alkaline decomposition of tungsten concentrate and sodium hydroxide, the product is reacted with hydrochloric acid, ammonium chloride solution, then went on with filtration, washing to get pure ammonium tungstate solution, and then carried out acid decomposition to get tungstic acid, and finally get product of tungstic after dehydration, drying and crushing. The reaction equations are as follows:
2. Hydrochloric acid decomposition method of tungstate. Heating the saturated aqueous solutions of sodium tungstate (Na2WO4), calcium tungstate (CaWO4) and other tungstate, then adding the solution slowly into boiling concentrated hydrochloric acid which is 2~3 times of the tungstate according to mole ratio, then the yellow tungstic acid is precipitated. At that time, when the adding speed is too fast or the temperature of solution is cooling down, it will easily to generate precipitation in forms of slurry or colloid which is causing trouble to the further treatment. After the tungstate solution is added, keep heating for 1 hour in the condition of water bath to change the precipitation into a state of easily being filtered, and then go on washing several times with 5% ammonium nitrate aqueous solution to clean out all of the Cl-, finally the tungstic acid is dried under temperature of 120℃. The reaction equations are as follows: